Fennel fruit, estragole
and the breast feeding mother.
David Hoffmann B.Sc., FNIMH
(thanks to David for sharing
this says jim)
Recent
concerns about the potential carcinogenicity of
estragole has led a number of regulatory bodies to call
for restrictions on the use of herbs that contain this
constituent. The range of issues raised are a microcosm
of the various concerns that appear at the interface of
traditional herbal medicine with the scientific method.
The following discussion will focus on the use of fennel
fruit and examine the clinical significance of any risk
posed by the traditional use of Apiaceae seed infusions
as galactagogues.
Estragole is a volatile phenylpropanoid found widely
distributed in plants of the Lamiaceae (Labiateae),
Apiaceae (Umbellifereae), Magnoliaceae, and other
families. The estragole is present as part of the
essential oil fraction where it is found with a range of
other constituents.
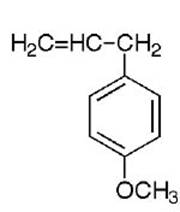
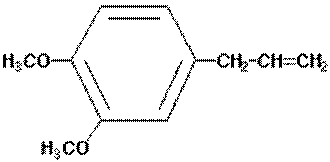
estragole
methyleugenol
An
number of medicinal plants produce essential oils that
contain estragole, including:
Acorus calamus (calamus)
Artemisia dranunculus (tarragon)
Cymbopogon citratum (lemongrass)
Foeniculum vulgare (fennel)
Hyssopus officinalis (hyssop)
Illicium verum (Chinese star anise)
Illicium anisatum (Japanese star anise)
Melilotus officinalis (sweet clover)
Myristica officinalis (nutmeg)
Ocimum basilicum (basil)
Ocimum marjorana (marjoram)
Petroselinum crispum (parsley)
Pimenta racemosa (allspice)
Pimpinella anisum (anise oil)
Piper betel (betel nut)
Recent
toxicological reviews have focused on the
carcinogenicity of estragole, for example those
published by the Reproductive and Cancer Hazard
Assessment Section in the Office of Environmental Health
Hazard Assessment of the California EPA[i],
and the International Program on Chemical Safety for the
WHO.[ii]
This
has led to some governmental regulatory agencies issuing
guidelines for the use of some herbs that contain this
constituent. For example the German
Federal Institute for Health
Protection of Consumers and Veterinary Medicine (BgVV)
advises that the content of the plant constituents,
estragole and methyleugenol, in foods should be reduced
as far as possible on precautionary grounds.[iii]
They state, for example:
-
It is not possible to estimate the actual scale of
the risk to the consumer from the regular
consumption of estragole or methyleugenol-containing
foods. It is, however, unlikely to be very high
given the relatively small intake amounts of these
substances. No studies have been produced up to now
which confirm a concrete risk to health in man.
-
For precautionary reasons, BgVV does however
recommend that consumers restrict any ongoing and
regular consumption of the above spices and herbal
teas which goes beyond their occasional use in the
kitchen. This applies in particular to fennel teas
which are frequently given to children to treat
wind. Tea preparations of this kind should only,
therefore, be administered over longer periods after
consulting a doctor or pharmacist.
-
Even if the grounds for suspicion, as in the case of
methyleugenol and estragole, are not sufficient in
order to justify a ban of traditional foods, the
consumer is at least given an opportunity to adapt
his personal consumption behavior to his individual
precautionary needs.
Similarly the Working Party on Herbal Medicinal Products
of the European Agency for the Evaluation of Medicinal
Products issued a Public Statement on the use of Herbal
Medicinal Products Containing Estragole in April 2005.[iv]
Note the following:
-
… it is concluded that the present exposure to
estragole resulting from consumption of herbal
medicinal products (short time use in adults at
recommended posology) does not pose a significant
cancer risk.
-
Nevertheless, further studies are needed to define
both the nature and implications of the dose
response curve in rats at low levels of exposure to
estragole. In the meantime exposure of estragole to
sensitive groups such as children, pregnant and
breastfeeding women should be minimized.
Estragole carcinogenicity
No
studies of the long-term health effects of human
exposure to estragole have been reported, in fact there
is no human evidence pointing to a problem.
Consider the following brief review of findings
concerning cancer and estragole. Several studies have
demonstrated the carcinogenic effects in mice. The mouse
liver metabolizes estragole to the potentially
carcinogenic compounds 1’-hydroxyestragole and
1’-sulphoxyestragole. These metabolites are potential
carcinogens because of their ability to bind to DNA in
vivo.[v]
This
process is associated with an increased chance of
genetic mutation.[vi]
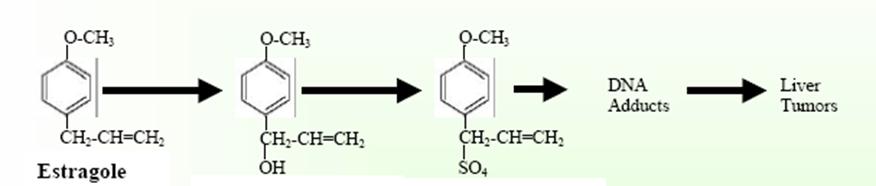
1’-hydroxyestragole 1’-sulphoxyestragole
Estragole administered to adult or new-born mice of
different strains produced malignant liver tumors:
-
Administration of estragole to adult female CD-1
mice via the diet for 12 months induced increased
incidences of hepatocellular carcinomas compared
with control mice.[vii]
-
Administration of ten doses of estragole by oral
intubation to newborn CD-1 mice produced increased
incidences of liver tumors in males, but not
females.[viii]
-
Estragole administered by multiple intraperitoneal
or subcutaneous injections to newborn male CD-1 mice
or multiple intraperitoneal injections to male
B6C3F1 mice resulted in high incidences of
hepatocellular carcinoma.[ix]
- A
single intraperitoneal dose of estragole
administered to newborn male B6C3F1 mice was also
found to be sufficient to induce a high incidence of
liver cancer.[x]
-
1’-Hydroxyestragole also induced high incidences of
liver tumors when administered by subcutaneous
injection to newborn CD-1 mice or via
intraperitoneal injection to newborn male CD-1,
B6C3F1, CeH/HeJ, or C57B1/6J mice, or in the diet
for 12 months to adult female CD-1 mice.[xi]
The
carcinogenicity of estragole has not been investigated
in the rat, although one subcutaneous
injection study of derivatives of estragole in male rats
did not observe any treatment-related increases in
tumors.
From
this it can be concluded that estragole has demonstrated
carcinogenicity in mice and as such is a cancer hazard
to mammals. However what is the risk posed to humans?
The same genotoxic mechanism of
action was postulated for estragole as for safrole,
because both constituents metabolize to
1’hydroxyestragole and 1’-sulphoxy-estragole. Even
though both metabolites occur in human metabolism, there
are considerable quantitative differences between
phenylpropanoid metabolism in humans and. rodents.
The
profiles of metabolism, metabolic activation, and
covalent binding are dose dependent and the relative
importance diminishes markedly at low levels of exposure
(i.e. these events are not linear with respect to dose).
In
particular, rodent studies show that these events are
minimal probably in the dose range of 1-10 mg/kg body
weight, which is approximately 100-1000 times the
anticipated human exposure to this substance.
For
these reasons it is concluded that the present exposure
to estragole resulting from consumption of herbal
medicinal products (short time use in adults at
recommended posology) does not pose a significant cancer
risk.
Fennel fruit and Estragole
The
issues raised in this article have come to the forefront
because of statements made in one of the authoritative
guides referred to by nurses and lactation consultants.
In the eleventh edition (2004) of Medications and
Mothers’ Milk, A manual of Lactational Pharmacology,
by Thomas W. Hale Ph.D., the entry for fennel has
changed from ‘moderately safe’ to ‘possibly hazardous’.[xii]
The following discussion of the important issues raised
is partially based on a paper published in German by
Iten and Saller.[xiii]
Unfortunately it is not availeble in an English
published form.
Fennel
seeds have a long history of use, as well as modern use
in herbal medicine, as a pleasant and effective
carminative and galactagogue. In central Europe there is
a tradition of ‘ lactation teas’ based on Apiaceae seeds
such as Fennel and Anise. Such lactation teas are sold
in German, Swiss and Austrian pharmacies as
"Traditionally Used Preparations." They are 'old drugs'
already on the market before the German Commission E
developed its new standards for herbal drug approval.
Compilations of relevant monographs include the
"Standardzulassungen für Fertigarzneimittel"[xiv]
(The German Standard Licenses for Finished Drugs), the
"Neue Rezeptformularium (NRF)"[xv]
(The New German National Formulary), which is published
by the "Arzneimittelkommission der Deutschen Apotheker"
(Drugs Commission of German Pharmacists), as well as
both the Austrian Pharmacopoeia[xvi]
(ÕAB) and the Swiss Pharmacopoeia[xvii]
(Ph.Helv.).
The
characteristic aroma of fennel seed is contributed by
the essential oil. This is obtained from the dried, ripe
fruit of Foeniculum vulgare var. vulgare
Thellung by steam distillation. The primary constituents
in the oil are trans-anethole (50-70%), (+)-fenchone
(9-22%) and estragole (2-5%) with many minor components.
Top of Form
Is there a risk posed by fennel
fruit because of estragole?
From the previous discussion it is
clear that estragole is a potential hazard (especially
to mice), but this does not immediately define the
nature of the risk posed (if any) to humans. To clarify
this the terms hazard and risk must be defined.
Hazard
refers to the potential toxicity of a substance in an
actual situation; how one deals with the substance in
that particular situation determines the risk. Risk
differs from hazard in that it cannot usually be
determined experimentally. It may be inferred from
epidemiological data, or it may be predicted from
mathematical models, but rarely can it be measured.
Instead, a more qualitative approach to identifying risk
must be adopted. The following defined terms are
routinely used in risk assessments.
-
Hazard is the capability of a substance to cause an
adverse effect, although the term can also be
applied to physicochemical properties, such as
flammability or explosiveness
-
Risk is a statistical term that indicates the
probability that the hazard will occur under
specific exposure conditions
-
Risk assessment is the process by which hazard,
exposure, and risk are determined
-
Risk management is the process of selecting the most
appropriate action based on the results of risk
assessment and social, economic, and political
concerns
In
order to determine whether a substance is toxic when
present in a large enough amount, several types of
information are used. The most conclusive information
comes from observation of cases in which humans are
exposed to the substance, either in clinical or
epidemiological studies. In the absence of information
from human studies, inferences are drawn from animal
studies, from in vitro studies using living cells, or
from comparisons to similar substances that pose known
hazards. As the source of the information moves further
from actual human studies, the uncertainty becomes
greater.
Can the extrapolation be made
from mice to humans? Or even mice to rats?
All the reports concerning
estragole toxicity, and hence generalizations concerning
herbs that contain it, are based on work with specific
laboratory strains of mice. In turn these tests involved
feeding very high doses of purified estragole. Dosage
ranged between 0.05 to 1,000 mg/kg.
It is
well established that considerable differences in
sensitivity to toxic substance among different species
occurs. For example, mice are more sensitive than rats
to the carcinogenic effects of 1,3-butadiene, a chemical
used in the production of synthetic rubber and other
resins that is one of the 189 hazardous air pollutants
identified in the Clean Air Act amendments.[xviii]
Metabolic pathways
Three different metabolic pathways have been identified
through which estragole can lead to potentially toxic
metabolites which can form DNA adducts.
It is believed that the initial step in chemical
carcinogenesis is the attachment of the chemical to DNA
to produce DNA adducts.
This covalent modification of DNA bases can alter the
structure and in turn, the biological processing of the
DNA by cellular proteins governing replication,
transcription and repair. If not repaired or repaired
incorrectly, these modifications may eventually lead to
mutations and ultimately cancer, especially if the
adduct is located in an oncogene or tumor suppressor
gene.
However, even though epoxides of
estragole form in vitro DNA adducts, such adducts
are not found in humans because of rapid detoxification
via epoxide hydrolases and glutathione transferases.[xix]
Dose
dependence
Another important difference in
estragole metabolism between mice and humans is
highlighted by an examination of dose dependency. In
this case, the genotoxic metabolite found in urine,
1’-hydroxyestragole, can be used as a indicator of
interspecies differences. In mice increasing doses of
estragole leads to increasing levels of the metabolite
in urine.
- Low doses (0.05-50 mg/kg body
weight) led to 1.3-5.4% 1’-hydroxyestragole.
- High doses (500-1,000 mg/kg
body weigh), led to 11.4-13.7%1’-hydroxyestragole.
In humans, the amount of
1’-hydroxyestragole in the urine remained constant at
0.2-0.4% throughout a wide dosage range (1-250 mg
estragole or 0.01-5 mg/kg body weight).[xx]
A subsequent study on the metabolism of trans-anethole
found that it was eliminated by humans 6 to 9 times
quicker than by mice.[xxi]
A confounding issue is seeing
dosages in mice as having relevance in humans. The dose
given to mice amounted to 50 – 100 times the quantities
consumed by people normally. It has been estimated by
the Scientific Committee on Food of the European
Community that the daily exposure to estragole from food
in people is in the range of 4.3-8.7mg. Hager’s
Handbook states that 100ml of fennel tea, made from 6g
of fennel fruits and 450mL of water, contains only 0.4mg
estragole.[xxii]
Iten and Saller make a telling
point in that estragole containing plants are not a
normal component of the diet of mice. In humans, the
exposure to estragole is well established and has
developed over evolutionary time. The differences in
metabolism suggests that humans have adjusted over the
course of time to the naturally occurring amounts of
estragole in the context of a detoxification process.
Whole plant v. purified constituent
The next issue is a common
conceptual stumbling block. Can the properties of an
isolated constituent be assumed from a herb that the
chemical is found in. Here is not the place for a
detailed exploration of this issue, but consider these
points.
- In all the animal studies,
isolated, purified estragole was used. Thus the
findings give a toxicological profile of this
molecule. In humans, however, estragole usually
enters the body as a component of fennel tea, or as
a food that has been seasoned with an herb that
contains is, such as basil or tarragon. In this
context estragole occurs in the form of an extremely
complex phytochemical mixture.
- If single constituent in
vivo data can be used a basis for statements
about a herb, then data about other constituents
should also be considered. Fennel fruit contains a
whole range of antioxidant constituents, which be
considered protective agents against cancer
genesis.[xxiii]
For example anethole, the main component of the
essential oil, possess anti-inflammatory and
anti-carcinogenic actions.[xxiv]
Toxicological studies of fennel fruit found no
evidence that the complex mixtures obtained by
ethanolic extracts had any carcinogenic potential,
even at very high single doses (0.5, 1 and 3 g/kg
body weight), and followed-up with application of
100 mg extract/kg body weight/day, over a 90 day
period.[xxv]
Thus doubt remains that data from
animal experiments can be extrapolated to humans, either
those concerning desired effects or those concerning
undesired effects (e.g. side effects, toxicity).[xxvi]
In other words an authoritative risk assessment about
fennel necessitates human data. This would include
clinical studies, epidemiological and experimental data.
Consideration of these issues
(dose, administration form, and differences in
metabolism between species) raises doubts about the
conclusion that fennel seed can be ‘reasonably
anticipated to be a human carcinogen’.
Recognizing these critical issues,
the European Agency for the Evaluation of Medicinal
Products (EMEA) published a new evaluation of estragole
and methyleugenol as constituents of phytomedicines.[xxvii]
According to the EMEA, the use of estragole-containing
phytopharmaceutical products in typical measured doses
and durations of use presents no significant risk of
cancer. They recommended that these medicines not be
restricted outright for children or for women during
pregnancy or breastfeeding, but rather to minimize their
use.
[i]
http://www.oehha.ca.gov/prop65/pdf/estragf.pdf
[ii]
http://www.inchem.org/documents/jecfa/jecmono/v14je08.htm
[v]
Phillips DH, Miller JA, Miller EC, Adams B
(1981). Structures of the DNA adducts formed in
mouse liver after administration of the
proximate hepatocarcinogen 1'-hydroxyestragole.
Cancer Res44 41:176-186.
[vii]
Miller EC, Swanson AB, Phillips DH, Fletcher TL,
Liem A, Miller JA (1983). Structur activity
studies of the carcinogenicities in the mouse
and rat of some naturally occurring and
synthetic alkenylbenzene derivatives related to
safrole and estragole. Cancer Res. 43(3):1124-34.
[x]
Wiseman RW, Miller EC, Miller JA, Liem A (1987).
Structure-activity studies of the
hepatocarcinogenicities of alkenylbenzene
derivatives related to estragole and safrole on
administration to preweanling male C57BL/6J x
C3H/HeJ F1 mice. Cancer Res 47(9):2275-83.
[xi]
Drinkwater NR, Miller EC, Miller JA, Pitot HC
(1976). Hepatocarcinogenicity estragole
(1-allyl-4-methoxybenzene) and
1'-hydroxyestragole in mutagenicity of
1'-acetoxyestragole in bacteria. JNCI
57(6):1323-31.
[xii]
Hale TW,Medications
and Mother's Milk: A Manual of Lactational
Pharmacology Pharmasoft Medical Pub; 11th
(April 01 2004)
[xiii]
Iten F, Saller R:
Fennel Tea: Risk Assessment of the Phytogenic
Monosubstance Estragole in Comparison to the
Natural Multicomponent Mixture.
Forschende Komplementärmedizin und Klassische
Naturheilkunde / Research in Complementary and
Classical Natural Medicine 2004;11:104-108 [in
german]
[xiv]
Braun, R.
et al., eds. 1997. Standardzulassungen für
Fertigarzneimittel: Text und Kommenter.
Stuttgart, Germany: Deutscher Apotheker Verlag.
[xv]
Arzneimittelkommission der Deutschen Apotheker
(AKDA). 1995. Neues Rezeptur-Formularium (NRF).
Stuttgart, Germany: Deutscher Apotheker Verlag.
[xvi]
Österreichisches Arzneibuch (ÖAB). 1991.
(Austrian Pharmocopoeia). Wein, Österreich:
Verlag der Österrichischen
Staatsdruckerei.
[xvii]
Pharmacopoeia Helvetica (Ph. Helv. VII). 1994
(Swiss Pharmacopoeia, 7th ed.).
Bern, Schweiz: Verlag Eidgenössische Drucksachen
und Materialzentrale.
[xviii]
Himmelstein MW Turner
MJ Asgharian B et.al.. Comparison of blood
concentrations of 1,3-butadiene and butadiene
epoxides in mice and rats exposed to
1,3-butadiene by inhalation. Carcinogenesis
(1994);15(8):1479-1486.
[xix]
Guenthner TM, Luo G: Investigation of the role
of the 2',3'-epoxidation pathway in the
bioactivation and genotoxicity of dietary
allylbenzene analogs. Toxicology 2001;160:47–58.
[xx]
Sangster SA, Caldwell J, Hutt AJ, Anthony A,
Smith RL: The metabolic disposition of
[methoxy-14C]-labelled trans-anethole, estragole
and p-propylanisole in human volunteers.
Xenobiotica 1987; 17:1223–1232.
[xxi]
Caldwell
J, Sutton JD: Influence of dose size on the
disposition of trans-[methoxy- 14C]anethole in
human volunteers. Food Chem Toxicol 1988;26:
87–91.
[xxii]
Brand N: Foeniculum; in Blaschek W, Ebel S,
Hackenthal E, Holzgrabe U, Keller K, Reichling J
(Hrsg): Hager ROM 2002. Hagers Handbuch der
Drogen und Arzneistoffe. Berlin, Springer, 2002.
www.hagerrom.de.
[xxiii]
Parejo I, Viladomat F, Bastida J, Rosas-Romero
A, Flerlage N, Burillo J, Codina C: Comparison
between the radical scavenging activity and
antioxidant activity of six distilled and
nondistilled mediterranean herbs and aromatic
plants. J Agric Food Chem 2002;50:6882–6890.
[xxiv]
Chainy GB, Manna SK, Chaturvedi MM, Aggarwal BB:
Anethole blocks both early and late cellular
responses transduced by tumor necrosis factor:
Effect on NF-kappaB, AP-1, JNK, MAPKK and
apoptosis. Oncogene 2000;19:2943–2950.
[xxv]
Shah AH, Qureshi S, Ageel AM: Toxicity studies
in mice of ethanol extracts of
Foeniculum
vulgare
fruit and
Ruta chalepensis
aerial
parts. J Ethnopharmacol 1991;34:167–172.
[xxvi]
Pound P,
Ebrahim S, Sandercock P, Bracken M B, Roberts I:
Where is the evidence that animal research
benefits
humans? BMJ 2004;328:514–517.
[xxvii]
European Agency for the Evaluation of Medicinal
Products: Final position paper on the use of
herbal medicinal products containing estragole.
www.emea.eu.int/pdfs/human/hmpwp/033803en.pdf.
|

